The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with real or potential tissue damage or described in terms of such damage”. Regardless of the severity of the pain, it always requires a response, the faster the better. But the pain is chronic, intolerant, like in cancer patients, does not respond to taking “standard” non-narcotic analgesics or medications from the “grandmother’s first aid kit”. Such patients are forced to take stronger drugs for their analgesic effect, most often opioids.
Medications that reduce or relieve pain are called analgesics. The modern classification of analgesics involves dividing them into four main groups:
- narcotic (opioid) analgesics;
- non-narcotic (non-opioid) analgesics;
- analgesics of a mixed type of action;
- drugs of other pharmacological groups that have an analgesic effect.
Everyone has heard something about opioids, but most likely, most people have associations associated with the abuse of these substances. But we are not interested in the recreational effect of the Papaver somniferum alkaloid, but its medical use.
Perhaps, everyone knows the “world-class star” among the group of narcotic analgesics. Meet me, morphine. The apothecary Friedrich Wilhelm Serturner, a twenty-year-old young man at that time, can be considered his father without a twinge of conscience. In the laboratory of his father, who was fond of the art of alchemy, as it was fashionable at that time, the young Serturner received all the skills for subsequent discovery. After the death of his father, he begins to experiment with various substances in the court pharmacy in Paderborn. Since opium was covered with an aura of mystery, then, of course, Serturner did not ignore it with his attention. The isolated powder was boldly tested on all dogs running past the pharmacy. The dogs did not mind and after being treated with an admixture of magic powder, they fell into a deep sleep without feeling the pinches of the Serturner . The young scientist immediately realized that a substance with similar properties could acquire great importance for humanity. Having made a number of experiments on himself, Serturner named it morphine in honor of the Greek god of sleep. This happened in 1804. You know the rest of the story. From centuries of use and excitement to legislation to restrict the use of opioids and the emergence of black markets.
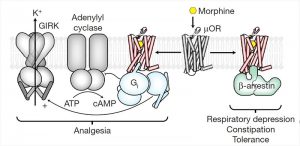
Positive and negative effects of opioids
The easiest way to understand the mechanism of action of opioids is to know that an opioid is a substrate that excites certain receptors. There are five types of opioid receptors in modern pharmacology, the most studied of which are μ, δ, and κ. All opioids interact to some extent with different types of opioid receptors, but there are the most typical agonists and antagonists for each type of opioid receptors. There are a lot of effects realized through these receptors, they are all very interesting and affect a person, if not at the organizational level, then certainly at the multi-organ level (starting with the central nervous system, ending with the urinary system). The pronounced activity of opium is manifested more through the effect on the m-receptors .
μ-receptors are divided into subtypes. There are three of them in total, and different effects are realized through the impact on a certain subtype. The effect of the ligand on the mk1-receptor will lead to an analgesic effect. At the same time, physical tolerance to opium drugs develops through this subtype of the receptor. When the ligand interacts with the mk2-receptor subtype, side effects occur: respiratory depression up to apnea, decreased peristalsis in the gastrointestinal tract, physical and mental dependence. In addition, there may be such effects as depression of the cardiovascular center in the medulla oblongata, oligo – or anuria, nausea, vomiting, constipation and many other very undesirable things. The function of the μ3-receptor is not yet known.
The main effect of interest to us — analgesic-is realized through the suppression of the activity of the structures of the central nervous system. These structures are located at different levels and perform a controlling (restrictive) function in relation to pain stimuli. They can be divided into 3 levels:
subcortical structures — near-conducting gray matter, reticular formation, suture nuclei;
hypothalamus;
the cortex of the large hemispheres.
Also, the analgesic effect is carried out through a decrease in the excitability of the emotional and vegetative centers of the hypothalamus, the limbic system and the cerebral cortex, which leads to a weakening of the negative emotional and mental assessment of pain .
Endogenous opioids
As for the analgesic effect, then opioids are simply great and have surpassed many! It is always interesting to reveal the secrets of those who do something perfectly. The secret of opioids was revealed at the end of the last century. First, the receptors in the brain that responded to the effects of opiates were discovered. Then there was one of the outstanding achievements in neurology-the discovery of the neural mechanism of action of opiates . These studies led to the discovery of a class of chemicals secreted by the brain, called enkephalins, and later to the discovery of endorphins. All these are morphine-like endogenous substances (endogenous opioids). Endorphins have a rather long path of formation: it all starts with proopiomelanocortin (POMK), which is produced in the anterior and intermediate lobes of the pituitary gland and in some other tissues (intestines, placenta). After the magical transformations of POMK into adrenocorticotropic hormone (ACTH) and β-lipotropin, a different set of peptides, including endorphins, is formed in different cells from these precursors.
Imagine that! Each of us has our own excellent system of protection against any pain, any experiences, any negative phenomena. After all, endogenous opioids, as well as exogenous ones, bind to opioid receptors and realize the effect of analgesia. That’s right, but not quite.
After the discovery of endorphins, attempts were indeed made to obtain their synthetic analogues, since it has now become clear that opioids are not such an evil, but, as is usually the case with drugs, a double-edged sword. It was assumed that such compounds would be powerful painkillers, free from the adverse effects associated with the use of narcotic drugs: after all, it is the human body’s own product. Unfortunately, the search was not crowned with success. The analgesic effect of the obtained substances was weaker than that of morphine. And if scientists tried to make the effect comparable in terms of pain relief with exogenous opiates, they got serious side effects as a result .
Why did this happen? Let’s remember that our body has a system for ensuring homeostasis. Everyone from school remembers what it is. You can even chorus: the ability of the body to maintain the constancy of the internal environment. So, in a normal physiological state, there is a balance between synthesis, release, binding to the receptor and the reverse capture of the neurotransmitter, the result of which is a sense of inner comfort. What is important, the body itself does not produce an excessive amount of endogenous opioids, as this can lead to a number of side effects, which have already been mentioned (addiction, respiratory depression up to apnea, nausea, constipation, etc.). Thus, one of the types of homeostasis is carried out in the human body — the so-called state of “opioid sufficiency”. If a substance that can contact the opioid receptor enters the body from the outside, then this condition is violated.
Who determines the result
The highest concentration of µ-receptors was found in the caudate nucleus. In high concentrations, these receptors are present in the cortex, thalamus, and hypothalamus. They are also found in moderate amounts in the parotid gray matter, the body of the stomach, in the duodenum, in the ileum and in smaller quantities in other places. These receptors (GPCRs ) are located on the cell membrane and interact through the G-protein with the membrane enzyme. G-protein is a universal mediator in the transmission of signals from the receptor to the enzymes of the cell membrane that catalyze the formation of secondary mediators of the hormonal signal. When the opioid hits the receptor, the G-protein is activated, changing its conformation, and actively interacts with the membrane enzyme. The result is a change in the speed and activity of processes in the cell.
Biomolecula has repeatedly written about the wonderful GPCRs: “The Nobel Prize in Chemistry (2012): for the receptors of our first, third and fourth senses”
The interaction of the opioid with the M-receptor leads to conformational changes not only of the G-protein, but also turns the receptor itself into a substrate for protein kinase. The ligand-activated receptor is phosphorylated by serine or threonine residues . Β-arrestins bind to the activated and phosphorylated receptor. Here is the one we need! It is the β-arrestins that “decide” whether there will be a side effect from taking an opioid substance. The proof of the above was provided by studies on mice. It was found that if morphine is injected into mice deprived of m-receptors, they will have neither analgesic effect nor side effects, in particular, respiratory depression. The scientists did not stop there and investigated what would happen in mice without β-arrestins 1 and 2. It turned out that when morphine was administered to such mice, they had an analgesic effect, and it was stronger and longer than in mice with β-arrestins 1 and 2. But, what is noteworthy, there was no respiratory depression, constipation and other negative manifestations. The conclusion was obvious. We need to continue working towards the study of β-arrestins.
The arrestin protein family includes four proteins. Arrestins 1 and 4 are expressed in the rods and cones of the retina, respectively. Arrestins 2 and 3 (also known as β-arrestins 1 and 2) are present in all tissues. They control the activity of G-protein coupled receptors at three levels:
silencing-separation of the receptor from its G-protein;
internalization — removal of the receptor from the cytoplasmic membrane, re-return to the membrane and / or degradation;
conducting signal activation or inhibition of intracellular signaling pathways independently of G-proteins.
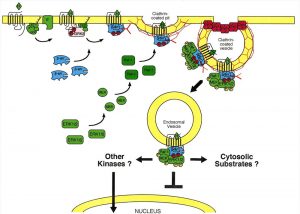
The managerial abilities of β-arrestin provide clathrin-dependent endocytosis — the entry of fragments of the cytoplasmic membrane together with all its contents into the cell in the form of bubbles covered from the outside with a lattice of polymerized clathrin. Clathrin is a protein that has the ability to form structures with an ordered grid, they are also called clathrates. The formed vesicle, inside which the receptor is subjected to endocytosis, and the further course of events can develop in different ways.
The beginning of a detailed study of opioids can be counted from the above-mentioned discovery of Serturner in 1804. Much has been clarified since then, but the specific molecular mechanism of the occurrence of side effects is still being discussed. One thing is recognized by all scientists without exception: whether or not there will be a negative effect in the form of respiratory depression, reduced peristalsis in the gastrointestinal tract, physical and mental dependence and other effects depends on β-arrestin. There are three main hypotheses for the implementation of this dependence. They appeared gradually, but they could not replace and exclude each other. Therefore, we will try to understand all three assumptions. I would like to emphasize that the hypotheses are not intended to exclude each other. Perhaps all the mechanisms take place, because complex processes are found everywhere in the human body.
Hypotheses that work
The first hypothesis (the youngest by origin) is the most reasonable and understandable. It states that β-arrestins 1 and 2 stimulate intracellular molecular signals independently of G-proteins and further cascades associated with G-protein. β-arrestins can activate the mitogen — protein kinase cascade. The basis of this cascade is MAP kinases-serine/threonine-specific protein kinases that, in response to extracellular stimuli, regulate cellular activity (gene expression, mitosis, differentiation, cell survival, apoptosis, etc.).
After the ligand-opioid has joined the μ-receptor, this complex binds to β-arrestin. At the same time, the immersion of the receptor complex into the cell begins with the formation of an endosome. The resulting complex (GPCRs + ligand-opioid + β-arrestin) is able to further bind to the MAP kinase. There are several signal paths associated with this system, but one works here. This system is the ERK (extracellular signal-regulated kinase) pathway, which includes a chain of activations and interactions of ERK1 / 2 proteins with other kinases, resulting in the signal passing into the cell nucleus. Here, the processes of transcription and further expression of the corresponding molecules are already taking place, thanks to which the cell can respond to external stimuli in one way or another. The function of such a mechanism is not fully understood.
Interestingly, this signaling paradigm has been confirmed in some GPCRs (for example, in angiotensin GPCRs), but has not yet been confirmed in µ-opioid receptors. Scientists suggest that if such a mechanism is peculiar to one type of GPCRs, then it also applies to other systems. We can try to assume that the intracellular processes implemented through the MAP kinase cascade lead to the following changes: after the intracellular processes mediated through MAP kinases, the expression of certain molecules that regulate ion exchangers occurs, mainly affecting potassium and calcium channels. This is how all the subsequent effects in the body, which were mentioned, are realized-through the activation of potassium and inhibition of calcium channels. It is necessary that a large amount of potassium is released from the cell according to concentration gradients, after which hyperpolarization of the membrane occurs. This condition is also called an inhibitory postsynaptic potential. It is obvious that at this time the cell is not capable of generating an action potential and conducting impulses. There is an inhibition of those processes in which this cell takes part. This is how the effect of anesthesia is realized (the cell is not able to respond to activating impulses from various neuropeptides responsible for conducting pain impulses), the effect of respiratory depression, the occurrence of constipation due to the suppression of peristalsis in the gastrointestinal tract.
In addition, it is known for certain that respiratory depression is partially realized through potassium channels of internal rectification (IRK). The experience of studying the effect of systematic infusion of opioids to mice with the absence of IRK showed that in the end, the opioid still led to a decrease in the respiratory rate and its subsequent depression. This shows that, after all, the implementation of effects is more complicated than everyone thought, and requires study. It is possible that these mechanisms are related to G-protein-independent signaling cascades, first suggested in studies with β-arrestin-deprived mice, which were mentioned earlier. In such a scenario, the elimination of β-arrestin or the weakening of the affinity of the receptor molecule with respect to it can level the signal transmission pathway and the subsequent biological response. Actually, mainly on the basis of this hypothesis, a new drug molecule PZM21 was obtained. We’ll talk about it later.
The second hypothesis is related to the fact that β-arrestin acts differently in different subtypes of the m-receptors (m1 and m2). The effect of the ligand on the mk1-receptor will lead to an analgesic effect, and the interaction of the ligand with the mk2-receptor will lead to the development of side effects. It seems logical for scientists that, accordingly, the m1-receptors are located in the nervous system (for example, in the near-conducting gray matter, reticular formation), and the m2-receptors are located in those areas in which they cause side effects. For example, the depression of the respiratory center is associated with the location of the mk2 receptors in the respiratory center. This hypothesis is currently considered insufficiently reliable and requires research. But still, the authors of articles even in 2016 mention it (although this hypothesis has existed for more than 30 years without a 100% evidence base), so we still believe in its implementation in practice.
The third hypothesis states that β-arrestin acts through other receptors, i.e. not through GPCRs. For example, on serotonin 5-HT4 receptors, affecting their activity in the neurons of the PBC complex (pre-Bötzinger complex). This complex is understood as a cluster of neurons in the ventrolateral region of the medulla oblongata. Together, they are responsible for generating the rhythm of breathing. Accordingly, the effect of respiratory depression is realized by the influence on this complex. Studies have been conducted in which scientists have shown that more than half of all 5-HT4 receptors in the PBC complex are interconnected with opiate µ-receptors in the same complex. These receptors, according to a mechanism not yet explained by scientists, are able to act as antagonists. The μ-receptor is activated — the activity of 5-HT4-receptors is antagonistically inhibited. The result of a cascade of subsequent events is the effect of respiratory depression. To test this hypothesis, studies were conducted with 5-HT4-receptor agonists. Their effect on these receptors led to a decrease in respiratory depression caused by opioids. But at the same time, interestingly, there was no loss of analgesic effect.
This hypothesis explains only the mechanism of one side effect. At the same time, it, like the previous hypotheses, is just a hypothesis that does not yet have 100% reliable evidence. It should be clarified that scientists do not give up and are not satisfied with the current state of affairs. For example, modern concepts claim that the actions of ERK1 / 2 (which were mentioned earlier in the first hypothesis) lead to inhibition of tolerance to opioids in the neurons of the near-conducting gray matter. Such studies indicate that the mechanism of action of opioids is not monosyllabic. Each cascade of signals, molecular pathways, and opportunities for interaction of molecules is important and carries information that together will give us a complete understanding of the problem. Knowing the essence of the problem, we will be able to solve it.
Is there a solution?
Opioid analgesics act in such a way that a sick person who is forced to take them quickly develops side effects. This makes us think about the expediency and legality of the use of opioids, which dramatically reduces their availability for patients.
Until recently, opioid analgesics, which do not yet have an alternative to pain relief in terms of their effectiveness, were a “headache” for scientists and doctors. The subject of the research was a special group of patients for whom all possible undesirable manifestations are only a grain compared to pain, which only opioids can cope with. In May 2016, at the second Russian conference on supportive therapy in oncology, one of the speakers noted that, although opioids are the drugs of choice for the treatment of chronic pain syndrome in the range from moderate to severe pain, Russia is still in last place in terms of their medical consumption among narcotic analgesics compared to other countries (Canada — 57.9%, the USA — 31.0%, Western Europe — 34.2%, Eastern Europe — 4.2%, the Baltic States — 2.3%, Russia — 0.5%). This is due to both legislative aspects and numerous negative factors: frequent admission (every 4 hours), the rapid development of tolerance, the need for frequent visits to the doctor, the difficulty of self-administration at home, the side effects mentioned many times, etc.
It is hoped that if not all, then most of the problems in the use of opioid analgesics will be solved soon. In 2016, the journal Nature published an article “Structure-based discovery of opioid analgesics with reduced side effects”, which describes an interesting and important study. The authors managed to get closer to solving a long-unsolvable, and already familiar, problem — to create a narcotic analgesic without the side effects inherent in this group of drugs. Through long-term mental and computer research, scientists tried to find a suitable molecule.
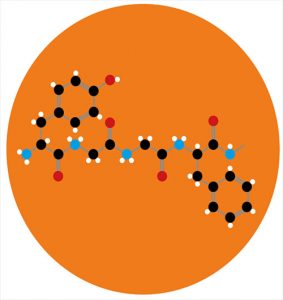
Initially, more than three million molecules were obtained that conformationally fit the structure of the μ-receptor. 2500 of the best compounds were then analyzed manually for interaction with key polar regions of the active center of the receptor. Of the selected 23 molecules, seven showed the greatest affinity for the m-receptor. The most highly selective compound was named PZM21 (remember the name — perhaps this is a future celebrity!).
This substance acts on the opioid μ-receptor as follows. Earlier it was said that β-arrestin attaches to the activated and phosphorylated GPCR (μ-receptor) after successive reactions. Its connection ensures the further course of events, the result of which is the occurrence of side effects. But PZM21 works in such a way that even after phosphorylation, activation and change of the GPCR conformation, β-arrestin does not attach to the receptor. This is due to a change in the conformation of the mk-receptor itself in favor of further activation of the G-dependent pathway, through which there are no side effects.
Thus, the above was confirmed by the experience with the presence of overexpressed GRK2 (G-protein-coupled receptor kinase2). This is a family of serine/threonine protein kinases that recognize and phosphorylate agonist-activated GPCRs. That is, the μ-receptor is phosphorylated after an opioid ligand is attached to it. Only this moment is waiting for beta-arrestin, ready “at full steam” to contribute to the realization of undesirable side effects. But the conformation of the μ-opioid receptor changes so that β-arrestin is not able to bind to it. And in the experiment it was shown that even under conditions of overexpression of GRK2 at the maximum concentration of PZM21, the content of β-arrestin still remains low. Conclusion: when using PZM21 as an opioid agonist, a chain of reactions is further formed not along the β-arrestin pathway, but along the pathway associated with the G-protein. As a result, this leads to a positive therapeutic effect (analgesia), which eliminates side effects in the form of respiratory depression, reduced peristalsis in the gastrointestinal tract, physical and mental dependence. The maximum analgesic effect of PZM21 in vivo lasted 180 minutes and at the same time without side effects. An interesting comparison was the effects of PZM21 and morphine. Thus, at the same dose of two substances, PZM21 caused an analgesic effect in 87% of mice after 15 minutes, morphine-in 92% of mice after 30 minutes.
The authors of the study still emphasize that, perhaps, some effects that are so positive compared to other opioid mk-receptor agonists occurred accidentally, therefore they require further numerous tests. In addition, whether such an unprecedented positive effect will be preserved in vivo in the conditions of a variety of reactions and all processes of the human body’s vital activity. What the metabolism, pharmacokinetics and pharmacodynamics of such a drug will be is still unknown for us.
The authors of the study indicate that the source of their inspiration was the previous experiments in the search for mk-receptor agonists. For example, in 2014, the article “Biased agonism of the μ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers” was published. We observed 30 healthy men, some of whom received a drug of the substance TRV130 (an agonist of the μ-receptors), some-placebo, some-morphine. Numerous indicators were studied, including the safety, tolerability and analgesic effect of TRV130. The analgesic effect of the drug was excellent even compared to the effects of morphine, but respiratory depression, albeit to a lesser extent, still occurred. Plus, a number of additional side effects were observed, for example, nausea.
In a 2016 study, scientists also compared the “newly made” PZM21 with TRV130 and morphine for respiratory depression. It was found out that morphine leads to apnea after 30 minutes, TRV130 induces temporary, transient depression, on average, for 15 minutes, but PZM21 does not affect the respiratory rhythm. Even if the problem of side effects of opioids is not solved, I want this study to serve as a basis for further discoveries. I especially want the research to concern not only the pharmacological effects and the synthesis of new molecules, but also the clarification of the above hypotheses. Still, reading a modern article, I do not want to see the phrase “the mechanism is not fully clarified”.
Conclusions
Pain can be treated in different ways: you can tolerate it and try to overcome it according to Immanuel Kant’s treatise “On the ability of the spirit to overcome painful sensations by the power of the will alone”. We can philosophize about it and speak in the words of Delia Guzman: “We should not fight with pain, but rather perceive it as a guiding light, as a kind of way to warn us and make us reconsider our actions and correct our actions.” You can consider pain as a function of a highly organized system and a protective reaction, but all this is left behind when you feel it yourself or see how someone else feels it. It is necessary to fight pain, it is necessary to take all possible measures to make life easier for a person, to improve its quality. Now it remains for us to observe further numerous clinical trials and studies of this extremely interesting and important discovery, perhaps to wait for new works related to blocking the effects of β-arrestin, and, perhaps, to participate in the discoveries ourselves. Everything is so that a person experiencing pain does not live according to the principle of the Count of Monte Cristo “wait and hope”, but lives a full life, as far as possible to include everything positive in this concept.